what will happen to the red blood cells in flask x?
8.4: Osmosis and Improvidence
- Page ID
- 58825
Learning Outcomes
- Define osmosis and diffusion.
- Distinguish amidst hypotonic, hypertonic, and isotonic solutions.
- Draw a semipermeable membrane.
- Predict behavior of blood cells in dissimilar solution types.
- Depict menses of solvent molecules beyond a membrane.
- Identify the polar and nonpolar regions of a cell membrane.
- Explain the components present in a phospholipid.
Fish cells, like all cells, take semipermeable membranes. Eventually, the concentration of "stuff" on either side of them volition fifty-fifty out. A fish that lives in salt water will accept somewhat salty water within itself. Put information technology in freshwater, and the freshwater will, through osmosis, enter the fish, causing its cells to swell, and the fish will die. What will happen to a freshwater fish in the ocean?
Osmosis
Imagine y'all have a cup that has \(100 \: \text{mL}\) h2o, and you add together \(15 \: \text{g}\) of table sugar to the water. The saccharide dissolves and the mixture that is at present in the cup is fabricated up of a solute (the sugar) that is dissolved in the solvent (the water). The mixture of a solute in a solvent is called a solution.
Imagine now that y'all accept a second cup with \(100 \: \text{mL}\) of h2o, and yous add \(45 \: \text{1000}\) of tabular array sugar to the water. Just like the first loving cup, the saccharide is the solute, and the water is the solvent. But now you have ii mixtures of unlike solute concentrations. In comparing two solutions of unequal solute concentration, the solution with the higher solute concentration is hypertonic, and the solution with the lower solute concentration is hypotonic. Solutions of equal solute concentration are isotonic. The offset sugar solution is hypotonic to the second solution. The 2d carbohydrate solution is hypertonic to the beginning.
You now add together the two solutions to a beaker that has been divided by a semipermeable membrane, with pores that are too small for the sugar molecules to laissez passer through, just are big enough for the water molecules to pass through. The hypertonic solution is ane one side of the membrane and the hypotonic solution on the other. The hypertonic solution has a lower water concentration than the hypotonic solution, and then a concentration slope of water now exists across the membrane. Water molecules will move from the side of college h2o concentration to the side of lower concentration until both solutions are isotonic. At this point, equilibrium is reached.
Ruby-red blood cells behave the same way (run into figure below). When blood-red blood cells are in a hypertonic (college concentration) solution, water flows out of the jail cell faster than it comes in. This results in crenation (shriveling) of the blood cell. On the other farthermost, a scarlet blood cell that is hypotonic (lower concentration outside the cell) will result in more water flowing into the jail cell than out. This results in swelling of the cell and potential hemolysis (bursting) of the jail cell. In an isotonic solution, the flow of water in and out of the cell is happening at the same charge per unit.
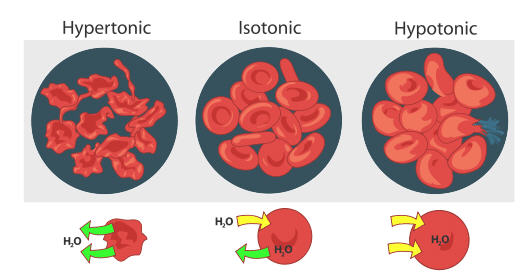
Osmosis is the diffusion of h2o molecules beyond a semipermeable membrane from an area of lower concentration solution (i.e., higher concentration of h2o) to an expanse of college concentration solution (i.east., lower concentration of h2o). Water moves into and out of cells past osmosis.
- If a prison cell is in a hypertonic solution, the solution has a lower h2o concentration than the prison cell cytosol, and h2o moves out of the prison cell until both solutions are isotonic.
- Cells placed in a hypotonic solution will take in water across their membranes until both the external solution and the cytosol are isotonic.
A red blood prison cell will peachy and undergo hemolysis (burst) when placed in a hypotonic solution. When placed in a hypertonic solution, a red blood cell will lose water and undergo crenation (shrivel). Animal cells tend to do best in an isotonic environment, where the menses of water in and out of the cell is occurring at equal rates.
Improvidence
Passive send is a way that small molecules or ions move across the cell membrane without input of energy by the prison cell. The three primary kinds of passive transport are diffusion (or simple improvidence), osmosis, and facilitated improvidence. Unproblematic diffusion and osmosis do not involve send proteins. Facilitated diffusion requires the assist of proteins.
Improvidence is the movement of molecules from an surface area of high concentration of the molecules to an area with a lower concentration. For cell ship, diffusion is the movement of small molecules beyond the cell membrane. The difference in the concentrations of the molecules in the ii areas is chosen the concentration gradient. The kinetic energy of the molecules results in random motion, causing diffusion. In elementary diffusion, this process proceeds without the aid of a ship protein. It is the random motion of the molecules that causes them to move from an area of high concentration to an area with a lower concentration.
Improvidence will continue until the concentration gradient has been eliminated. Since diffusion moves materials from an surface area of college concentration to the lower, it is described equally moving solutes "downwards the concentration gradient". The end result is an equal concentration, or equilibrium, of molecules on both sides of the membrane. At equilibrium, move of molecules does not cease. At equilibrium, in that location is equal movement of materials in both directions.
Non everything can make information technology into your cells. Your cells have a plasma membrane that helps to guard your cells from unwanted intruders.
The Plasma Membrane and Cytosol
If the outside environment of a jail cell is water-based, and the inside of the prison cell is also generally water, something has to make sure the cell stays intact in this surroundings. What would happen if a jail cell dissolved in water, like carbohydrate does? Obviously, the cell could not survive in such an environment. So something must protect the cell and permit it to survive in its water-based environment. All cells have a barrier around them that separates them from the surroundings and from other cells. This barrier is called the plasma membrane, or cell membrane.
The Plasma Membrane
The plasma membrane (meet figure beneath) is fabricated of a double layer of special lipids, known every bit phospholipids. The phospholipid is a lipid molecule with a hydrophilic ("h2o-loving") head and two hydrophobic ("water-hating") tails. Because of the hydrophilic and hydrophobic nature of the phospholipid, the molecule must be arranged in a specific pattern as only sure parts of the molecule tin physically exist in contact with h2o. Retrieve that there is h2o exterior the cell, and the cytoplasm inside the cell is mostly h2o also. And so the phospholipids are arranged in a double layer (a bilayer) to proceed the cell separate from its environment. Lipids do not mix with h2o (call up that oil is a lipid), so the phospholipid bilayer of the cell membrane acts as a barrier, keeping water out of the prison cell, and keeping the cytoplasm inside the cell. The cell membrane allows the cell to stay structurally intact in its water-based environs.
The role of the plasma membrane is to control what goes in and out of the cell. Some molecules can go through the jail cell membrane to enter and leave the cell, only some cannot. The cell is therefore non completely permeable. "Permeable" ways that anything can cross a barrier. An open up door is completely permeable to anything that wants to enter or get out through the door. The plasma membrane is semipermeable, meaning that some things tin enter the cell, and some things cannot.
Molecules that cannot easily laissez passer through the bilayer include ions and small hydrophilic molecules, such equally glucose, and macromolecules, including proteins and RNA. Examples of molecules that can easily diffuse across the plasma membrane include carbon dioxide and oxygen gas. These molecules diffuse freely in and out of the prison cell, along their concentration gradient. Though water is a polar molecule, information technology can also diffuse through the plasma membrane.

Cytosol
The inside of all cells also contain a jelly-like substance called cytosol. Cytosol is composed of h2o and other molecules, including enzymes, which are proteins that speed upwardly the cell's chemical reactions. Everything in the cell sits in the cytosol, similar fruit in a Jell-o mold. The term cytoplasm refers to the cytosol and all of the organelles, the specialized compartments of the prison cell. The cytoplasm does not include the nucleus. As a prokaryotic cell does not have a nucleus, the DNA is in the cytoplasm.
Contributors and Attributions
-
Allison Soult, Ph.D. (Department of Chemistry, University of Kentucky)
Source: https://chem.libretexts.org/Courses/University_of_Kentucky/UK:_CHE_103_-_Chemistry_for_Allied_Health_%28Soult%29/Chapters/Chapter_8:_Properties_of_Solutions/8.4:_Osmosis_and_Diffusion
Postar um comentário for "what will happen to the red blood cells in flask x?"